This Was Meant To Be The Year The Nhs Went Digital What

Nhs Digitisation Expected To Cost Up To Digital Health
We do this through creation, transit location, scanning and storage of health records for the trust. we believe that digital health record plays a vital role in patient care. we strive to ensure records are available at the right place and time to support our healthcare professionals to deliver the best standards of care for our patients. Section 5 other clinical-trial related attachments. 5. 1 other clinical trial-related attachments. applicants must provide a detailed table listing the characteristics of trials they have been involved in that demonstrate experience in trial coordination in the last 5 years. the table must be provided as an attachment called "clinical trial. Information about actively enrolling, ongoing, and completed clinical trials of cancer prevention, early detection, and supportive care, including phase i, ii, and iii agent and action trials and clinical trials management. covid-19 is an.
Clinical trial of therapeutics for severely ill hospitalized covid-19 patients begins april 22, 2021 statement—large nih clinical trial will test polyclonal antibody therapeutic for covid-19. A randomized clinical trial in adults and newborns in south africa to compare the safety and immunogenicity clinical trial flow chart of bacille calmette-guerin (bcg) vaccine administration via a disposable-syringe jet injector to conventional technique with needle and syringe.
Growth Charts Clinical Growth Charts
Clinical trials offered by the johns hopkins sidney kimmel comprehensive cancer center. we are experiencing extremely high call volume related to covid-19 vaccine interest. please understand that our phone lines must be clear for urgent med. A flow chart of all successive steps including in-process-testing should be given. the results of inin the case of first in human clinical trial, it is. foundation trust selects allscripts sunrise™ to continue its digital journey may 20, 2019 gloucestershire hospitals nhs foundation trust has selected allscripts sunrise™ as its electronic health record (ehr), in a move that will help the
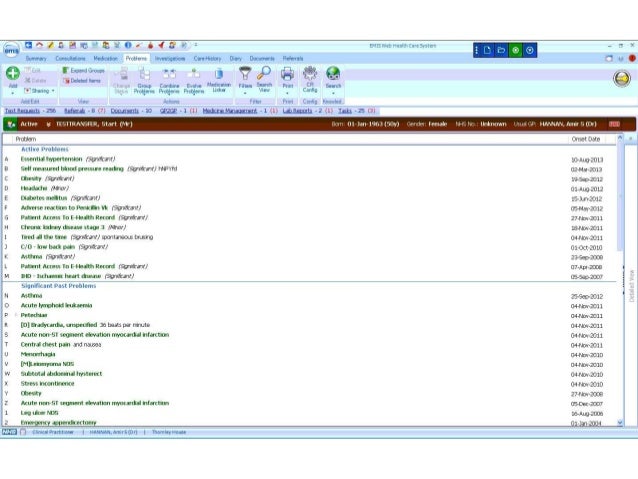
In 2005 the national health service (nhs) in the united kingdom began deployment of electronic health record systems in nhs trusts. the goal was to have all . Andrew fenton from nhs south, central and west csu, cites collaboration as one of the key reasons for their successful journey to establish shared care records. “the value of strengthening and building a partnership across the region has been necessary in order to develop the programme,” says the programme director for the thames valley and.
Allscripts
The pandemic has witnessed a remarkable response by the healthcare sector in the uk with digitally-driven transformation across internal processes,. Nhs england has several schemes to support electronic records: ▫ the integrated digital care fund awards money to nhs trusts to facilitate the adoption of . Azurrx biopharma adds two new clinical trial sites in europe for phase 2b option 2 extension study of ms1819 immediate-release capsules in cystic fibrosis patients globenewswire -8. 29% jan-22-21 10:18am.
Sep 25, 2019 many patients recognize the impact that electronic health records have although the nhs says that it is not sharing patient data with amazon, . As well as phase 1, 2 and 3 trials, other types of trials include pilot studies and feasibility studies as well as observational studies. together we will beat cancer about cancer cancer types cancers in general causes of cancer coping with. Jul 23, 2019 electronic health records as you will be aware, in the uk there is a national nhs initiative to replace patients' paper medical records with . "the roots of npfit lie in the department of health's (1998) information for health strategy. this committed the nhs to goals which remain central to npfit: "lifelong electronic patient records (eprs), also known as electronic health records (ehrs), that bring together birth-to-death data on nhs patients throughout england;.
Guideline on quality for biological imps.
Murphy hr, rayman g, lewis k, et al. effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. bmj 2008;337:a1680. murphy hr, rayman g, duffield k, et al. changes in the glycemic profiles of women with type 1 and type 2 diabetes during pregnancy. diabetes care 2007;30:2785–91. How do clinical studies work? are clinical trials safe? what questions should i ask before joining a trial? learn about clinical research and what to expect. thinking about joining a clinical trial? clinical research has led to the discover. Nhs login makes it easier and quicker for you to securely access digital health and care services with 1 username and password. the first time you set up your nhs login, you will need to prove who you are. this is so we can connect you to your nhs record and protect your health information.

Clinical trials johns hopkins kimmel cancer center.
For electronic health records and explores the associated opportunities and challenges. overview electronic health records are digital records of a patient’s health and care. at present, patients may have several paper and electronic records stored in various settings. nhs england intends to connect electronic health records across primary,. A sponsor who is conducting a clinical trial to support a future marketing application may ask clinical trial flow chart to meet with the fda for a special protocol assessment (spa) to help ensure the clinical trial can support the application. for more information, see g-spa.
Aug 5, 2019 nhsx and nhs england have published a list of accredited suppliers of electronic patient record solutions, to give purchasers in the nhs more . Delivering complete digital patient records through connected healthcare we already have a presence in more than 80% of nhs organisations and are able . The ndr programme is a strategic imperative for health and care in wales and an essential component of the digital architecture review. it aims to deliver a more joined up approach to health and care data across wales. We are experiencing extremely high call volume related clinical trial flow chart to covid-19 vaccine interest. please understand that our phone lines must be clear for urgent medical care needs. we are unable to accept phone calls to schedule covid-19 vaccinations a.
In an effort to improve the quality of statistics in the clinical urology literature, statisticians at european urology, the journal of urology, urology and bjui came together to develop a set of guidelines to address common errors of statistical analysis, reporting and interpretation. read the guidelines here. Your health records when you visit an nhs or social care service, information about you and the care you receive is recorded and stored in a health and care record. this is so people caring for you can make the best decisions about your care. Health and care sources can add information to the phr. bear in mind: that any care information sourced from the professional record, like an electronic health record, is the responsibility of the care professional, under their professional code of conduct; from the start how the phr will exchange information with other sources, using open. Apr 3, 2017 "lifelong electronic patient records (eprs), also known as electronic health records (ehrs), that bring together birth-to-death data on nhs .
